
Summer Project 1999
I am currently a summer intern through a program sponsered by the DOE at the Stanford Linear Accelerator Center (SLAC).This is the homepage of the place where I work. The Stanford Synchrotron Radiation Laboratory (SSRL) is a part of SLAC and it is where I work. The picture of the lab can be seen below.

This lab
consists of many different parts. The main part of the lab, SPEAR,
can be seen above. It is a ring where all the electrons and positrons
are stored. SPEAR
stands for Stanford Positron Electron Accelerating Ring. The other
ring is called the booster ring and it is where all the positrons and electrons
are made. As these particles circle the ring, they produce synchrotron
radiation beams (UV and x-ray photons). This is the world's most
intense x-ray source!! (WOW). It enables the study of matter at the
atomic or molecular scale of the surfaces of semiconductors or the structure
of proteins. SPEAR actually provided enough information for Burton
Richter to discover the fourth quark called the charm quark. This
discovery led him to win the nobel. ![]() prize
in physics in 1976. Pretty impressive don't you think?
prize
in physics in 1976. Pretty impressive don't you think?
Andrea
and I are working on a protein
crystallography project. She is also a fellow intern, chemistry
major, and fan of
Dawson's Creek.
Check out her homepage for more about her life and our project. She
does go to Harvard so her explanation might be better than mine.
| We do our crystal work in a cold room at a temperature of 38 degrees Fahrenheit. We wear fleeces to keep us warm (see caption to the right). Who knew that sunny California could be so cold? |  |
| The crystallization of proteins first involves the use of a gel to grease the wells of the tray (see picture). |  |
| The crystals are grown from crygogenic or non-cryogenic solutions. The cryogenic or non-cryogenic solutions are put into the wells of the tray. A screen of cryo or non-cryo solutions is sometimes used to provide a varitey of conditions for the crystals to grow. | 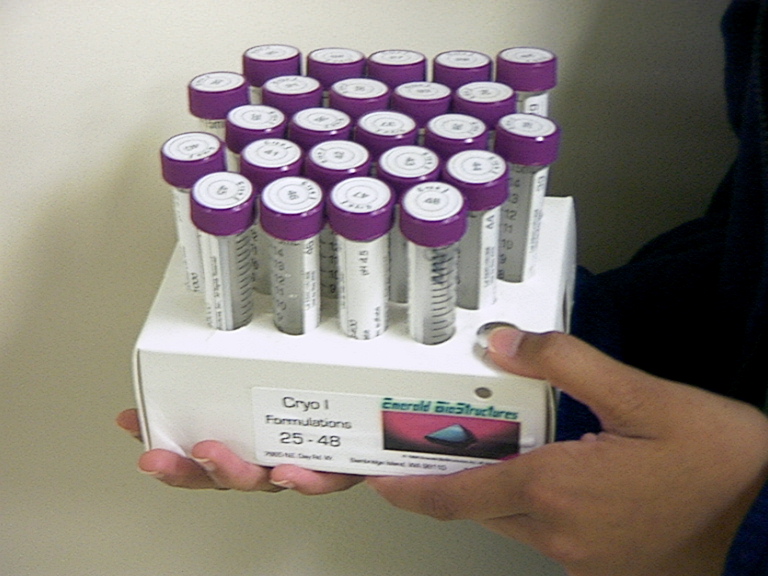 |
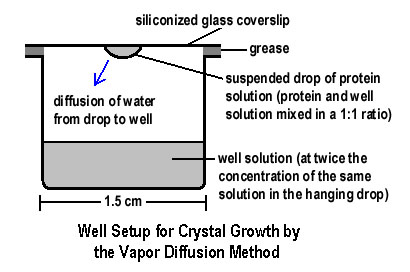
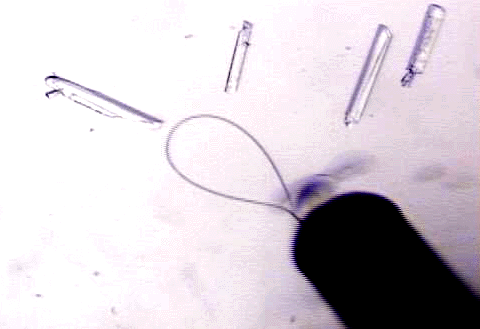
| The wells are covered by glass coverslips with a drop of the protein and the well solution. Tweezers are used to transport the coverslips to ensure that it covers the well appropriately. The protein crystals should grow in the drops, if the conditions are ideal. |
 |
|
|
 |
|
|
 |
|
|
|
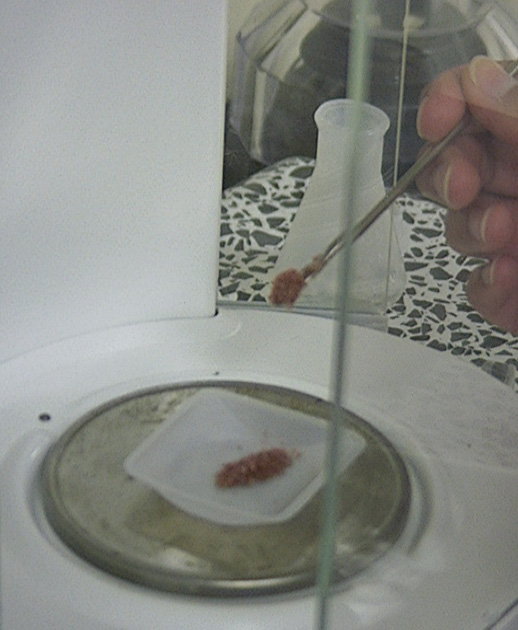
| X-rays that are provided by the Synchrotron Radiation are then used to probe the structure of the protein crystals. This information helps people to learn about the orientation of the electrons and other parts of the protein. The beamline, named 9-2, and the detector for the x-rays is shown here. From the data that the detector gives out, one can interpret the structure of the crystal. |  |
|
|
 |
... is to find the optimum conditions to allow the protein crystals to grow. The different "cryo" conditions are prepared by varying the pH, temperature, ionic strength, and buffer of the well solutions.
As our final duty in the SISE program, we had to give a 20 minute presentation
on our research this summer. This detailed deescription of protein
crystallography can be seen at Final
Powerpoint Presentation.